New Delhi: India’s apex drug regulator Central Drugs Standard Control Organization (CDSCO) will form a panel to examine whether more regulatory restrictions should be brought in place to curb the misuse of tapentadol, an opioid-based painkiller, and pregabalin, a medication widely used for managing nerve pain, ThePrint has learnt.
The decision to form the panel was taken last month.
In the case of tapentadol, the move comes after a request by the Union Department of Revenue, which administers the National Drugs and Psychotropic Substances (NDPS ) Act — a law prohibiting the production, possession, sale, and consumption of narcotic drugs and psychotropic substances.
Meanwhile, Punjab has complained about the growing misuse of pregabalin.
In the 62nd meeting of the Drugs Consultative Committee (DCC) — an advisory panel that comes under the CDSCO — last month, it was decided to form the sub-committee to decide on whether there should be more restrictions on the sale of the two drugs. The sub-committee will comprise regulators, pharmacologists, and psychiatrists.
The minutes of the meeting, a copy of which is with ThePrint, show that the Department of Revenue under the Ministry of Finance has asked the regulator to analyse tapentadol for its scheduling under the NDPS Act.
ThePrint reached Rajeev Singh Raghuvanshi, the Drug Controller General of India (DCGI), via calls for a comment. This report will be updated if and when a response is received.
Some experts have said that while introducing more restrictions on the supply of tapentadol and pregabalin may be a good idea, a lot will depend on the implementation.
“Tramadol (another opioid-based painkiller) is also restricted under the NDPS act, but the fact is that a lot of people can still access it purely for recreational purposes,” Dr Ravindra Rao, a professor of psychiatry at the National Drug Dependence Treatment Centre at Delhi’s All India Institute of Medical Sciences (AIIMS) and a researcher on opioids, told ThePrint.
Also Read: After small firms, India’s drug regulator puts big companies supplying to US, EU under scanner
Tapentadol
Tapentadol can be prescribed for moderate to severe pain, including pain caused by nerve damage from diabetes. ThePrint had reported how the problem of misuse of pharma opioids is growing in India due to the unregulated sale and purchase of these medicines.
Tapentadol is a controlled drug in many countries, including the US. The World Health Organisation (WHO) expert committee on drug dependence reviewed it in 2014 and recommended that owing to the insufficiency of data regarding dependence, abuse, and risk to public health, tapentadol may not be placed under international control but may be kept under surveillance.
In its October meeting, the DCC deliberated if tapentadol could be included in Schedule X of the Drugs and Cosmetics Rules, 1945, considering its misuse potential. Drugs on Schedule X cannot be purchased over the counter without a valid prescription of a Registered Medical Practitioner (RMP), and, according to the committee, this may help restrict its availability.
The panel further recommended an in-depth evaluation of the subject by a separate panel.
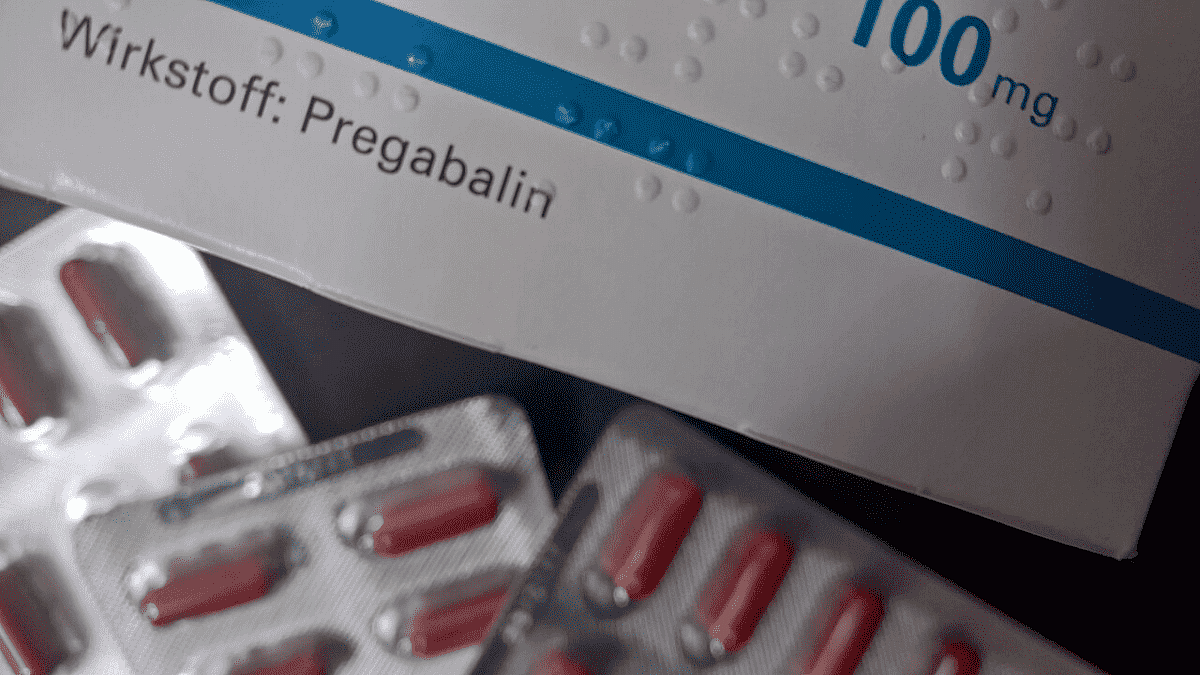
Pregabalin
The DCC said that the drug formulations — both tablets and capsules — containing pregabalin are available in different strengths in the market containing the drug from 75 mg to 300 mg/ unit dosage form.
“The drug formulations containing pregabalin are generally prescribed by the doctors for the management of neuropathic pain, to treat fibromyalgia,” the committee noted, adding that the tablets are used along with other medications to treat certain types of seizures in adults and children over 1 month, apart from managing nerve-related pains.
According to the panel, the Punjab Food and Drug Administration has reported that drug formulations containing it are being misused for intoxication and that these formulations are being seized from both licensed chemists and unlicensed premises.
As a solution, the panel has recommended including the drug under H1 of the Drugs Rules 1945.
H1 drugs are certain third- and fourth-generation antibiotics, specified habit-forming drugs, and anti-tuberculosis drugs whose sale has to be recorded with the name and address of the prescriber, the name of the patient, the name of the drug, and the quantity supplied.
Also, this record needs to be maintained for three years and be open for inspection. The rules also require a warning label on the packaging of the drug.
The advisory panel also said that as higher strengths of pregabalin don’t have much therapeutic usage and are being misused for intoxication, continuation of DCGI approvals of the dosage forms containing pregabalin 150 mg and 300 mg should also be re-evaluated.
(Edited by Uttara Ramaswamy)
Also Read: Modi govt likely to bring in stricter pan-Indian system to recall substandard drugs from market