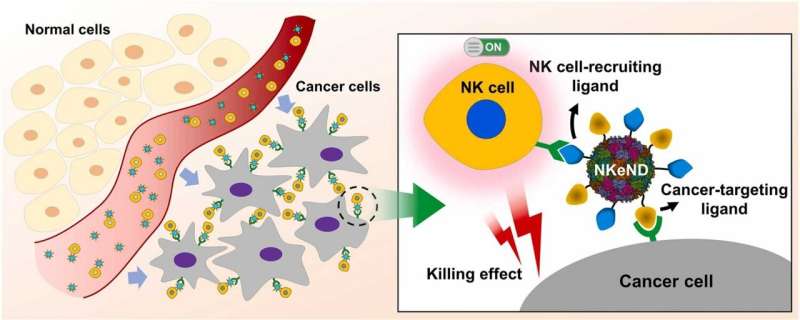
A study led by Professor Sebyung Kang and Professor Sung Ho Park in the Department of Biological Sciences at UNIST has unveiled a remarkable breakthrough in cancer treatment. The research team has successfully developed unprecedented “NK cell-engaging nanodrones” capable of selectively targeting and eliminating cancer cells, offering a potential solution for intractable types of cancers.
The innate lymphoid cells known as natural killer (NK) cells play a vital role in the body’s immune response against cancer. Numerous efforts have been made to harness the power of NK cells to develop effective cancer therapies. Now, the research team has designed and fabricated exceptional NK cell-engaging nanodrones, referred to as NKeNDs, using AaLS protein cage nanoparticles.
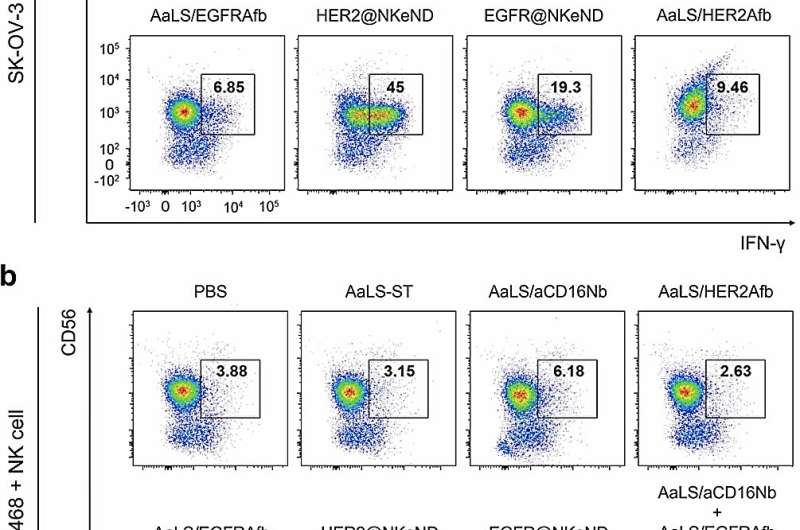
These groundbreaking NKeNDs simultaneously display cancer-targeting ligands, such as HER2Afb or EGFRAfb, and NK cell-recruiting ligands, aCD16Nb, on the surface of the AaLS through the SpyCatcher/SpyTag protein ligation system. The dual ligand-displaying NKeNDs, named HER2 @NKeND and EGFR@NKeND, have demonstrated the ability to selectively bind to HER2-overexpressing SK-OV-3 cells and EGFR-overexpressing MDA-MB-468 cells, respectively, as well as human NK cells.
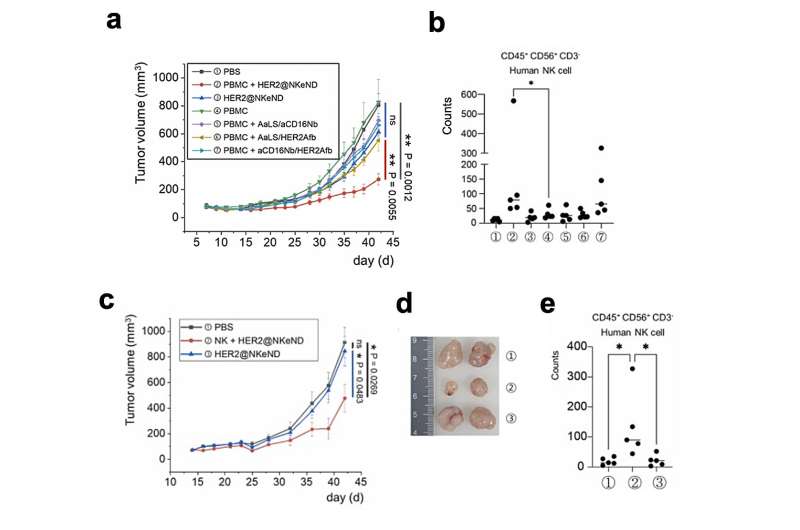
The physical engagement of human NK cells with the target cancer cells mediated by the NKeNDs activates the NK cells, enabling them to eliminate the target cancer cells in vitro effectively. Remarkably, in SK-OV-3 tumor-bearing mice, the administration of HER2 @NKeNDs along with human PBMCs facilitates the infiltration of activated human NK cells into the tumor sites. As a result, tumor growth is significantly suppressed without causing noticeable side effects.
This study showcases a novel approach to developing cancer-specific NK cell engagers by utilizing protein cage nanoparticles and recombinant cancer cell binders. It offers tremendous potential for the selective treatment of previously intractable types of cancers.
Professor Kang Se-byung expressed his excitement about the study, stating, “This research presents new possibilities for immune treatment through NK cell delivery nanodrones, overcoming challenges such as the movement and survival of NK cells. We aim to provide new opportunities for customized treatments that selectively address various types of cancer through further research, including cancer-specific immune cell induction.”
The study is published in the journal Nano Today.
More information:
Seong Guk Park et al, Protein cage nanoparticle-based NK cell-engaging nanodrones (NKeNDs) effectively recruit NK cells to target tumor sites and suppress tumor growth, Nano Today (2023). DOI: 10.1016/j.nantod.2023.102075
Citation:
Revolutionary nanodrones enable targeted cancer treatment (2023, December 29)
retrieved 30 December 2023
from https://phys.org/news/2023-12-revolutionary-nanodrones-enable-cancer-treatment.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

