Heterocyclic compounds are organic molecules with a ring structure comprising at least two or more elements. In most cases, these rings are composed of carbon atoms along with one or more other elements such as nitrogen, oxygen, or sulfur. They are highly sought after as raw materials in the chemical and pharmaceutical industry, owing to their versatility and excellent physiological activities.
While several methods are available for synthesizing these compounds, most of them involve high temperature and pressure conditions, or the use of precious metal catalysts, adding to the economic and environmental cost of producing heterocyclic organic compounds.
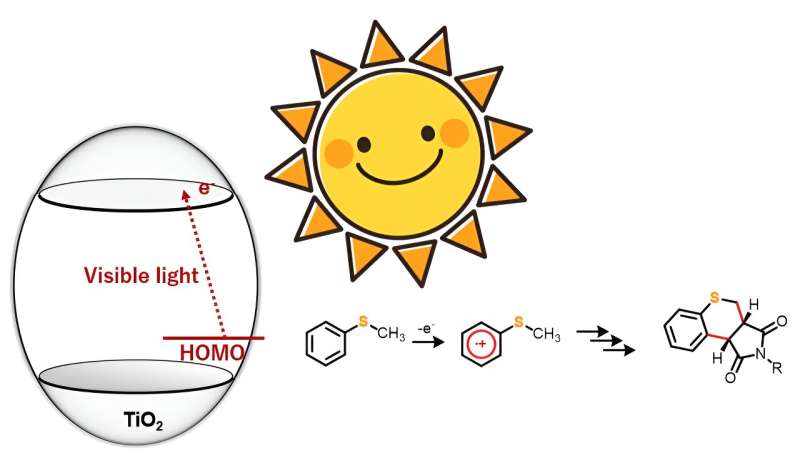
Now, however, a team of researchers from Japan and Bangladesh have proposed a simple yet effective method for overcoming these challenges. Their study was recently published in the journal Advanced Synthesis & Catalysis. Using the proposed strategy, the team demonstrated the synthesis of 20 sulfur-containing heterocyclic compounds in the presence of photocatalyst titanium dioxide (TiO2) and visible light.
The study was led by Professor Yutaka Hitomi from the Department of Applied Chemistry, Graduate School of Science and Engineering, Doshisha University, and co-authored by a Ph.D. candidate Pijush Kanti Roy from Doshisha University, Associate Professor Sayuri Okunaka from Tokyo City University, and Dr. Hiromasa Tokudome from Research Institute, TOTO Ltd.
TiO2 as a photocatalyst for driving organic reactions has captured the attention of synthetic chemists for a while now. However, many such processes require ultraviolet light to trigger the reaction. In this study, however, the research team found that under anaerobic conditions, sulfur-containing organic compounds like thioanisole derivatives, when hit with blue light, reacted with maleimide derivatives to form dual carbon–carbon bonds, yielding a new heterocyclic organic compound.
“We observed that while ultraviolet light generates highly oxidative holes, our approach allows for the selective one-electron oxidation of the substrate molecules using visible light. This approach can thus be employed in various organic chemical reactions,” explains Prof. Hitomi.
The researchers chose five 4-substituted thioanisoles and four N-substituted maleimides for the annulation or ring formation reactions. The team irradiated the starting material with blue light (wavelength > 420 nm) but observed no reaction. However, introducing TiO2 into the reaction system led to the synthesis of 20 different thiochromenopyrroledione derivatives with moderate-to-high yield. They found that within 12 hours of exposure to blue light, the reaction between thioanisole and N-benzylmaleimide led to the formation of a thiochromenopyrroledione derivative with 43% yield, which was close to the theoretical maximum yield of 50%.
The research team also observed substituent effect in the reactions to understand the corresponding mechanistic aspects. From the results, they postulated that the reaction proceeds through charge transfer from thioanisole to the conduction band of TiO2. Furthermore, they suggested that irradiation with blue light triggered one-electron oxidation of thioanisole, which further initiated the generation of α-thioalkyl radicals through deprotonation.
In summary, this new and refined approach demonstrates the potential of TiO2 for visible light photocatalysis for organic synthesis. It also provided crucial insights into the chemistry of complex heterocyclic compound synthesis. Moving forward, this approach can open up new possibilities for transitioning from current resource-intensive industrial chemical processes to a more energy-efficient system.
Prof. Hitomi says, “What drove our study was the desire to aid in the development of a sustainable chemical industry, and our findings appear to be a positive step in this direction.”
“We believe that the widespread adoption of this visible light-driven technology could assist in accessible and affordable synthesis of pharmaceuticals, with its profound impacts on the health and well-being of millions of people worldwide.” Thanks to the efforts of Prof. Hitomi and his team, their study has opened up new avenues in the field of organic synthesis, with the potential to revolutionize multiple chemical industries.
More information:
Pijush Kanti Roy et al, Blue Light‐Promoted Synthesis of Thiochromenopyrroledione Derivatives via Titanium Dioxide‐Catalyzed Dual Carbon–Carbon Bond Formation with Thioanisole and Maleimide Derivatives, Advanced Synthesis & Catalysis (2023). DOI: 10.1002/adsc.202301021
Citation:
Chemical synthesis using titanium dioxide: An eco-friendly and innovative approach (2024, January 1)
retrieved 1 January 2024
from https://phys.org/news/2024-01-chemical-synthesis-titanium-dioxide-eco-friendly.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

