BENGALURU: The effectiveness of drug molecules in treating various ailments is intricately linked to how well they navigate the complex internal environment of the human body. These properties, known as pharmacokinetic (PK) characteristics, determine the success of a drug as it traverses the digestive system, bloodstream, and biological barriers to reach its intended target.
In an illuminating study published in Nature Communications, researchers at IISc’s Molecular Biophysics Unit (MBU) have introduced an approach to enhance PK properties of “macrocyclic peptides,” a class of drug molecules highly sought after by the pharmaceutical industry worldwide.
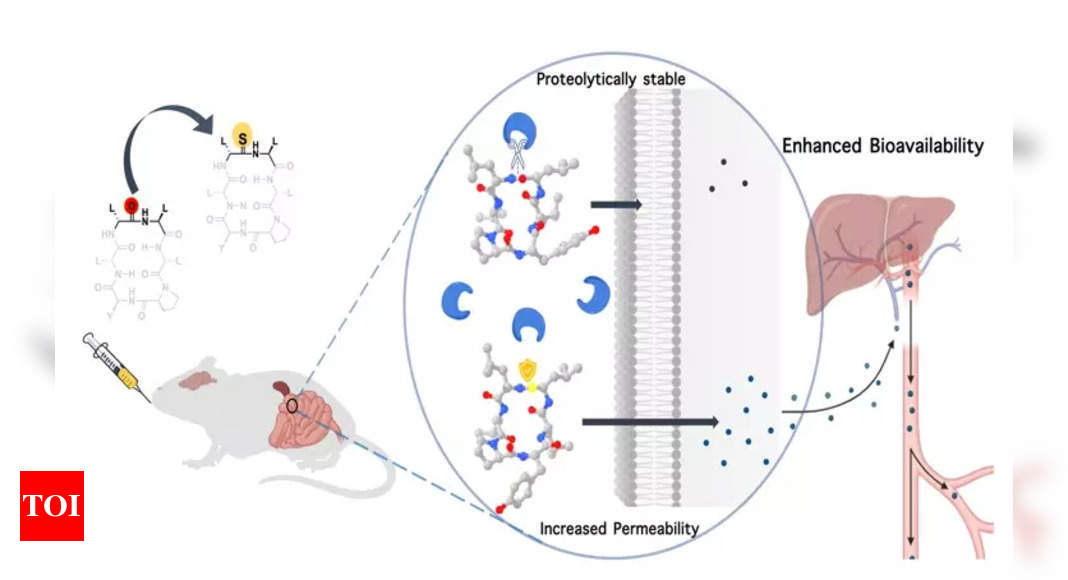
In a collaboration with Anthem Biosciences, IISc demonstrated that by substituting a single atom — oxygen with sulphur — in the backbone of a macrocyclic peptide, they could enhance its resistance to digestive enzymes and its permeability through cell membranes, significantly elevating its bioavailability.
“A significant portion of today’s medicinal treatments relies on small molecules administered orally in the form of pills. However, larger molecules, such as monoclonal antibodies, which tend to be more effective and specific, necessitate injection for delivery. As a middle ground, scientists have increasingly turned to macrocyclic peptides,” an IISc statement read.
These peptides, it added, consist of chains of amino acid residues bonded through amide bonds, forming circular structures that combine the favourable attributes of both small and large pharmaceutical molecules.
Like any protein, macrocyclic peptides are highly susceptible to degradation by digestive enzymes. And, for peptides to effectively traverse a lipid membrane, they must reduce their interaction with water and become more lipid-loving (lipophilic).
Professor Jayanta Chatterjee from MBU, the study’s corresponding author, highlighted the existing lack of concrete methods to enhance PK properties of macrocyclic peptides, aside from a chemical modification on biological regulation called the “N-methylation”.
Pritha Ghosh, a former PhD student at MBU and the first author of the study, said N-methylation entails replacing a hydrogen atom from the amide bond with a methyl group. While this modification prevents hydrogen bond formation with surrounding water, it can affect the peptide binding to its target, making it overly flexible and less specific.
In response to this challenge, Chatterjee and his team focussed on the oxygen atom within the amide bond, which is known to interact with water via hydrogen bonds. Through chemical synthesis of cyclic peptides, they discovered that replacing this oxygen atom with sulphur significantly increased the peptide’s lipophilicity, thus enhancing its permeability through lipid membranes.
Additionally, this modification rendered the peptide less susceptible to digestive enzymes, which typically target the oxygen atom in the amide bond – a site now fortified with sulphur.
To validate whether such a modified compound could retain its biological function, the team employed a shorter version of somatostatin, a pancreatic hormone that inhibits the body’s growth hormone. They substituted the oxygen atom in an amide/peptide bond with sulphur. The results were promising, as the modified somatostatin not only exhibited an extended presence in the bloodstream when injected under the skin of model animals but also effectively inhibited the growth hormone.
Ghosh emphasises that their research is not limited to somatostatin and will continue to explore other biologically active molecules. The oxygen-to-sulphur modification can be employed in combination with other strategies, possibly yielding even better results. This innovative technology can be harnessed to produce peptides with enhanced pharmacological properties, offering promising avenues for the pharmaceutical industry.
In an illuminating study published in Nature Communications, researchers at IISc’s Molecular Biophysics Unit (MBU) have introduced an approach to enhance PK properties of “macrocyclic peptides,” a class of drug molecules highly sought after by the pharmaceutical industry worldwide.
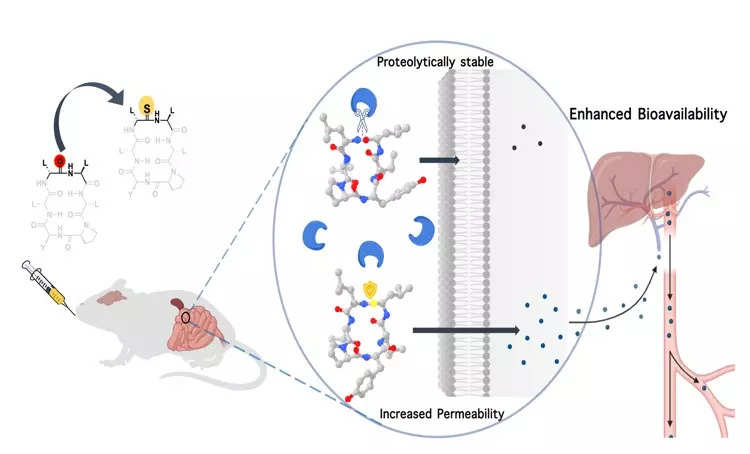
In a collaboration with Anthem Biosciences, IISc demonstrated that by substituting a single atom — oxygen with sulphur — in the backbone of a macrocyclic peptide, they could enhance its resistance to digestive enzymes and its permeability through cell membranes, significantly elevating its bioavailability.
“A significant portion of today’s medicinal treatments relies on small molecules administered orally in the form of pills. However, larger molecules, such as monoclonal antibodies, which tend to be more effective and specific, necessitate injection for delivery. As a middle ground, scientists have increasingly turned to macrocyclic peptides,” an IISc statement read.
These peptides, it added, consist of chains of amino acid residues bonded through amide bonds, forming circular structures that combine the favourable attributes of both small and large pharmaceutical molecules.
Like any protein, macrocyclic peptides are highly susceptible to degradation by digestive enzymes. And, for peptides to effectively traverse a lipid membrane, they must reduce their interaction with water and become more lipid-loving (lipophilic).
Professor Jayanta Chatterjee from MBU, the study’s corresponding author, highlighted the existing lack of concrete methods to enhance PK properties of macrocyclic peptides, aside from a chemical modification on biological regulation called the “N-methylation”.
Pritha Ghosh, a former PhD student at MBU and the first author of the study, said N-methylation entails replacing a hydrogen atom from the amide bond with a methyl group. While this modification prevents hydrogen bond formation with surrounding water, it can affect the peptide binding to its target, making it overly flexible and less specific.
In response to this challenge, Chatterjee and his team focussed on the oxygen atom within the amide bond, which is known to interact with water via hydrogen bonds. Through chemical synthesis of cyclic peptides, they discovered that replacing this oxygen atom with sulphur significantly increased the peptide’s lipophilicity, thus enhancing its permeability through lipid membranes.
Additionally, this modification rendered the peptide less susceptible to digestive enzymes, which typically target the oxygen atom in the amide bond – a site now fortified with sulphur.
To validate whether such a modified compound could retain its biological function, the team employed a shorter version of somatostatin, a pancreatic hormone that inhibits the body’s growth hormone. They substituted the oxygen atom in an amide/peptide bond with sulphur. The results were promising, as the modified somatostatin not only exhibited an extended presence in the bloodstream when injected under the skin of model animals but also effectively inhibited the growth hormone.
Ghosh emphasises that their research is not limited to somatostatin and will continue to explore other biologically active molecules. The oxygen-to-sulphur modification can be employed in combination with other strategies, possibly yielding even better results. This innovative technology can be harnessed to produce peptides with enhanced pharmacological properties, offering promising avenues for the pharmaceutical industry.
Denial of responsibility! Swift Telecast is an automatic aggregator of the all world’s media. In each content, the hyperlink to the primary source is specified. All trademarks belong to their rightful owners, all materials to their authors. If you are the owner of the content and do not want us to publish your materials, please contact us by email – swifttelecast.com. The content will be deleted within 24 hours.