A research team, led by Professor Zhang Jinsong from the Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Sciences, revealed insights into the mechanism by which the membrane protein MHZ3 collaborates with the ethylene receptor to regulate the phosphorylation of OsCTR2 (CONSTITUTIVE TRIPLE RESPONSE2), thereby controlling the switch of the ethylene signaling in rice.
The paper is published in the journal Nature Communications.
Plants cannot evade unfavorable environments by moving like animals do. Instead, they adapt to changing environments by dynamically regulating the levels of hormones within their bodies.
Ethylene is a classic gaseous hormone present in plants that can freely diffuse between cells, tissues, and individuals. Short bursts of ethylene may enhance plant adaptability, whereas prolonged ethylene exposure can be detrimental to plant growth and development. These characteristics of ethylene suggest that the initiation and termination of ethylene signaling should be a rapid process.
However, the understanding of early events in ethylene signaling remains limited.
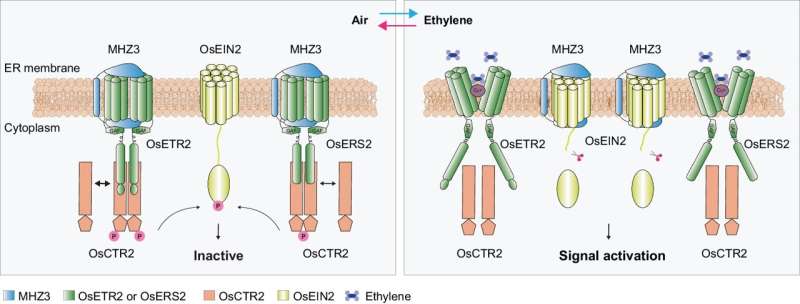
Zhang’s team utilized the rapid phospho-shift of OsCTR2, a negative regulator of rice ethylene signaling, in response to ethylene as a sensitive readout for signal activation. Analysis of an ethylene-insensitive rice mutant mhz3 revealed that MHZ3 interacts with ethylene receptors to promote the phosphorylation of OsCTR2.
Epistasis analysis indicated that MHZ3 and ethylene receptors are interdependent in facilitating OsCTR2 phosphorylation. MHZ3 and ethylene receptors co-localize on the endoplasmic reticulum membrane.
In air, MHZ3 interacts with both subfamily I and subfamily II ethylene receptors to stabilize the binding of ethylene receptors with OsCTR2, promoting the attachment of OsCTR2 to the endoplasmic reticulum membrane, and thereby maintaining OsCTR2 phosphorylation activity and turning off ethylene signaling.
Ethylene treatment disrupts the binding within the MHZ3-receptor-OsCTR2 complex, reduces OsCTR2 phosphorylation, and initiates downstream ethylene signaling.
Previous research by the same team discovered that MHZ3 is a stabilizing factor for the positive regulator OsEIN2 in the rice ethylene signaling pathway, playing a positive regulatory role. This study further found that MHZ3 interacts with ethylene receptors to regulate OsCTR2 phosphorylation, thereby participating in early events of ethylene signaling and playing a negative regulatory role.
The study elucidated the dual role of MHZ3 in the ethylene signaling pathway. In the absence of ethylene, MHZ3 interacts with ethylene receptors to maintain OsCTR2 phosphorylation, keeping OsCTR2 active and ethylene signaling off.
In the presence of ethylene, the interaction between MHZ3 and receptors weakens, leading to OsCTR2 deactivation, while MHZ3 redirects and stabilizes OsEIN2, keeping ethylene signaling active.
This research provides important insights into the early events of the ethylene signaling cascade and may also offer a conceptual paradigm applicable to other signaling pathways.
More information:
Xin-Kai Li et al, Membrane protein MHZ3 regulates the on-off switch of ethylene signaling in rice, Nature Communications (2024). DOI: 10.1038/s41467-024-50290-4
Citation:
Research unravels dual role of membrane protein in rice ethylene signal transduction (2024, August 23)
retrieved 23 August 2024
from https://phys.org/news/2024-08-unravels-dual-role-membrane-protein.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

