New Delhi: India’s apex drug regulator has given conditional approval to continue the manufacture and sale of five fixed-dose combination (FDC) medicines, asking drugmakers to produce post-marketing safety and efficacy data on three combinations, and to change the dosage and information label in the case of two others.
This comes seven months after the Union health ministry banned 14 FDC drugs stating that they lacked therapeutic justification.
FDC drugs are those that contain a combination of two or more active pharmaceutical ingredients (APIs) in a single form, which is usually manufactured in a fixed proportion.
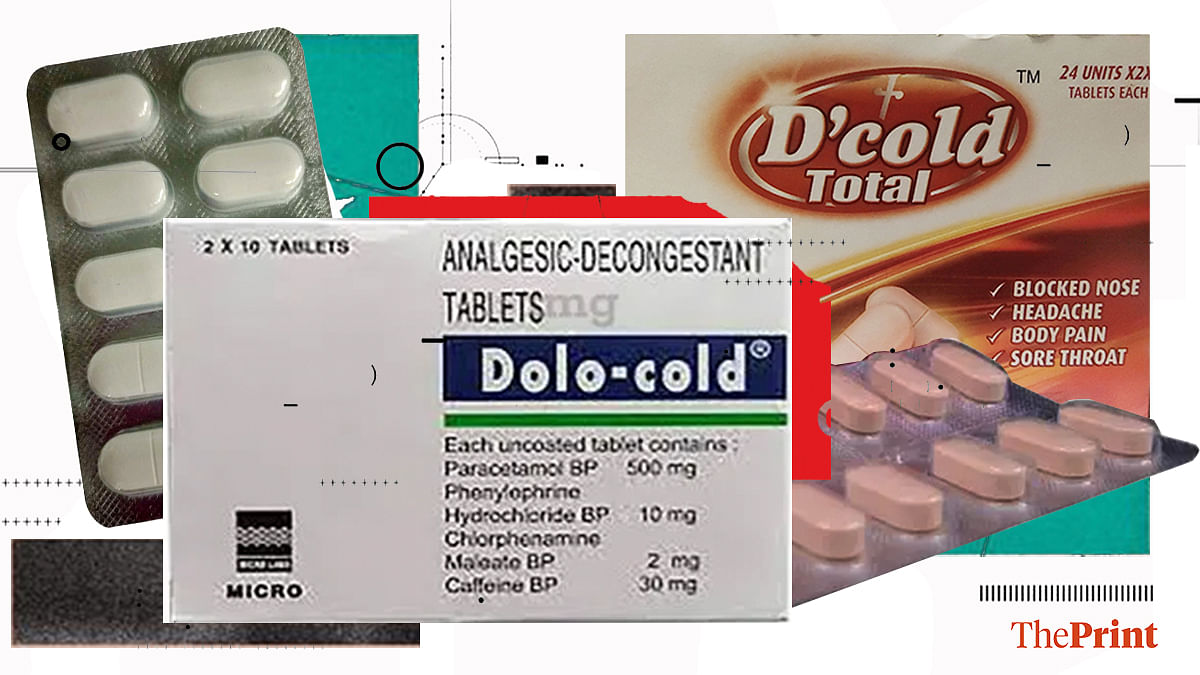
In an order issued Thursday, the Central Drugs Standard Control Organisation (CDSCO) has asked drug manufacturers to produce safety and efficacy data within one year in case of paracetamol + phenylephrine hydrochloride + caffeine anhydrous, and caffeine anhydrous + paracetamol + phenylephrine hydrochloride + chlorpheniramine maleate — the drugs sold for treating cold and allergy symptoms.
In the case of the second FDC, the regulator has instructed that the manufacturing companies should carry out randomised comparative phase IV clinical trials, comparing the combination with the individual ingredients in it.
“Accordingly, phase IV clinical trials (post-marketing) are required to be conducted to generate the data within the time frame of one year,” says the order undersigned by the Drug Controller General of India, Rajeev Singh Raghuvanshi, who heads the CDSCO.
Some of the popular brands using these combinations include D Cold Total and Dolo Cold.
ThePrint reached Reckitt and Micro Labs — the marketers of these drugs — for comment over email but did not receive a response till the time of publishing this report. This report will be updated if and when a reply is received.
ThePrint reached Raghuvanshi over phone for comment, but did not get a response. This report will be updated if and when a response is received.
The third combination for which the regulator has sought post-marketing surveillance data is paracetamol + propyphenazone + caffeine, which was earlier used in a popular tablet for treating headaches, Saridon.
The latest version of Saridon available in India does not have propyphenazone but the other two components.
The CDSCO order has also said that the combination of imipramine hydrochloride + diazepam has to be indicated for comorbid anxiety conditions and the duration of treatment should not exceed 6 to 8 weeks.
In case of chlorpheniramine maleate + ammonium chloride + sodium citrate — a combination used to treat cough with mucus — the specified dose strength should be followed and dose schedule for adults and children should be clearly mentioned without exceeding the maximum permissible dose, the apex drug regulator has instructed.
This instruction, says the order, has been given because drugmakers are manufacturing the FDC in different strengths.
Also Read: 70% of antibiotic fixed-dose combination drugs sold in India unapproved or banned, finds study
Chequered past of FDCs
The five FDC drugs part of the new CDSCO circular, like the 14 FDCs banned last year, were among 344 drug combinations that the government had first banned in 2016 after an expert panel, constituted at the behest of the Supreme Court, had declared them as “irrational” and found that they were marketed to patients without scientific data proving their efficacy and safety. The panel was headed by Dr C.K. Kokate.
The committee had noted that all drugs in India are required to secure marketing approval from the CDSCO before obtaining a manufacturing licence from state drug administrations.
But in the case of FDCs, the companies had obtained manufacturing licences from states without getting marketing licences from the CDSCO, which required them to submit a therapeutic justification for the drug.
While most drugs — out of the list of 344 originally banned for sale and marketing in India — were forced out of the market following the committee’s recommendation, some pharma companies contested the government’s ban order in the Delhi High Court for 19 drugs, saying that these were launched prior to 1988 when the rule mandating marketing approval by the CDSCO for every drug came into force.
Eventually, the health ministry in 2021, following instructions from the court, formed another panel under the chairmanship of Dr M.S. Bhatia, professor of psychiatry at University College of Medical Sciences in Delhi, to examine the case of these 19 drugs.
“The health ministry’s decision to ban 14 FDCs last year and the latest decision to allow continued marketing of five other FDC drugs are based on the recommendation of this panel,” a CDSCO official told ThePrint.
The new order may come as a breather for drugmakers but some experts have questioned the rationale behind it.
“The Kokate committee had earlier found these drugs to be irrational after noting there was no scientific data to prove that they worked — in that case it is difficult to understand how these drugs are being permitted to continue in the market,” said Chinu Srinivasan, a pharmaceutical expert who was instrumental in fighting the case against FDC drugs in the apex court. Srinivasan is associated with the patient rights group All India Drug Action Network.
Srinivisan also questioned how the Bhatia panel had allowed the combination of paracetamol + propyphenazone + caffeine, when the molecule propyphenazone — after being linked with serious adverse events such as heart attacks in some cases — had been banned in countries such as Singapore and Turkey.
(Edited by Nida Fatima Siddiqui)
Also Read: Antibiotic resistance is the newest ‘silent killer.’ Move over Covid