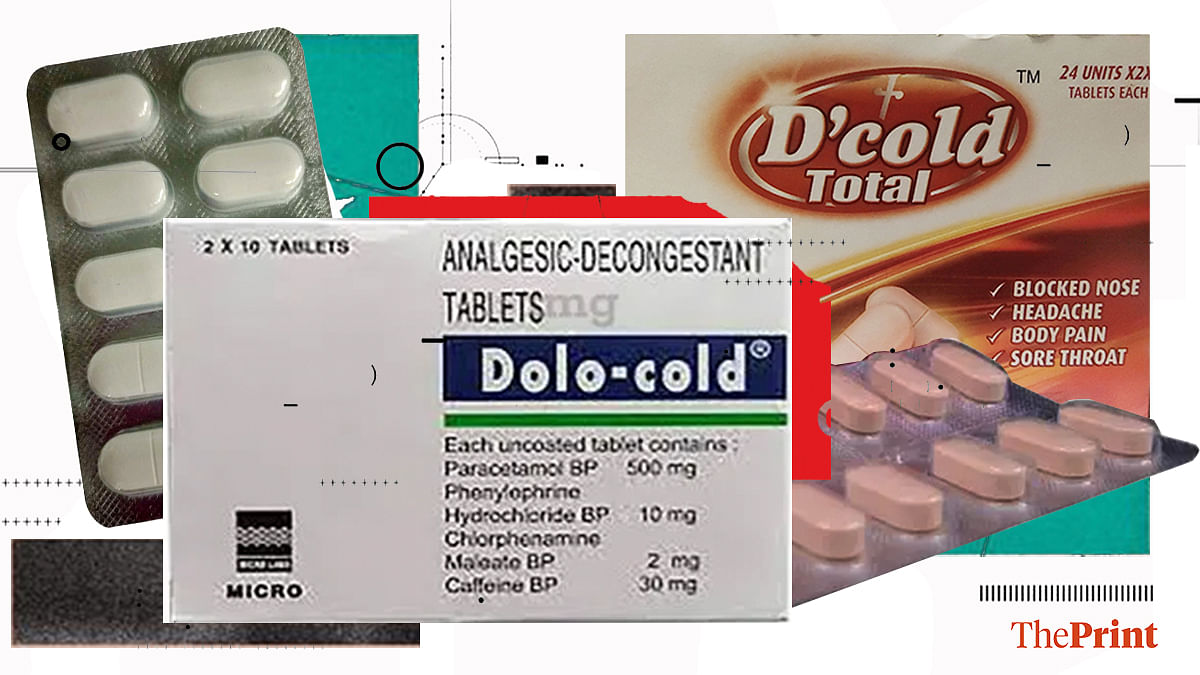
Drug regulator gives conditional nod for sale of 5 FDCs, seeks efficacy & safety data for 3 of them
New Delhi: India’s apex drug regulator has given conditional approval to continue the manufacture and sale of five fixed-dose combination (FDC) medicines, asking drugmakers to produce post-marketing safety and efficacy data …